Microtubule-kinetochore attachment formation
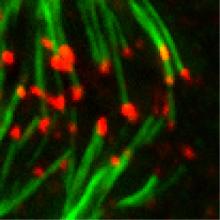
One key requirement for successful cell division in mammalian cells is the correct segregation of the duplicated chromosomes. For efficient chromosome segregation, it is essential that microtubules emanating from the two opposing spindle poles attach correctly to the kinetochores of sister chromatids. Initial, tentative attachments have to be converted to stable attachments that are able to withstand the tension created by the interplay of pulling forces from the spindle poles and resistance from sister chromatid cohesion. The formation of microtubule-kinetochore attachments as well as the development of tension across the kinetochores is monitored by the spindle assembly checkpoint (SAC), a multi-layered signal transduction machinery that stalls cell cycle progression in case of faulty microtubule-kinetochore attachments. Once all the kinetochores are successfully attached, the SAC has to be silenced to allow anaphase entry. We are interested in understanding how microtubule-kinetochore attachment formation and SAC signaling are integrated. Our lab has identified the astrin-kinastrin complex as a novel microtubule plus-end tracking activity that contributes to the fomation of stable microtubule-kinetochore attachments (Dunsch et al., 2011). We are currently analysing the targeting and the regulation of the astrin-kinastrin complex in the different phases of the cell cycle.